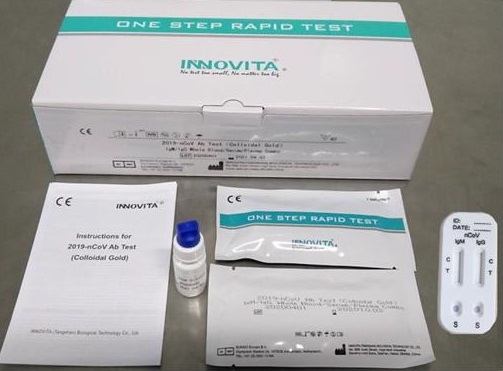
2019-Ncov Antibody Test (Colloidal Gold) kit manufactured by INNOVITA
₱ 1,800
Special corporate price and group discounts are available
- Pre-screening
- Doctor Consultation
- Test Interpretation
- Watch the Video to understand our Rapid Test
- Sign a waiver and consent to proceed on registration by filling up personal information and medical history
- Wait for a call or an email for approval.
FAQs
Frequently Asked Questions
What is Rapid Testing for COVID-19?
It is qualitative detection of the antibody IgM/IgG to novel Coronavirus (COVID-19) in human whole blood/serum/plasma.
What brand of Rapid Test kit will West Metro Medical Center will be using?
West Metro Medical Center will be using the 2019-Ncov Antibody Test (Colloidal Gold) kit manufactured by INNOVITA (Tangshan) Biological Technology Co. Ltd.
How accurate is your COVID- 19 rapid test?
The brand 2019-Ncov Antibody Test (Colloidal Gold) kit has a high sensitivity and specificity to bind to nucleic acid of IgG and IgM antibodies utilizing the immuno-capture method that indicates the presence of 2019-corona virus antigen. It is safe and FDA approved. This Rapid Test has a limited use, as a basis for the diagnosis and exclusion of COVID-19. It can be used as a supplementary detection tool.
How do I prepare to take the test?
Before a Rapid Antibody Test can be done for corporate accounts or walk-in, a pre-screening activity shall be conducted in order to be categorized as either Asymptomatic or at least Moderately Symptomatic.
Who can be tested using rapid COVID-19 test kits?
Anyone can take the test provided they are asymptomatic or patients that do not show any visible symptoms like dry cough, fever, diarrhoea and Mild Symptomatic or patients that show only mild symptoms may proceed with rapid test while those who are at least Moderately Symptomatic shall immediately follow the PCR Swabbing process.
How long will it take to get results from COVID-19 rapid test?
Blood extraction between 8:00 am to 12:00 noon, results available on or before 5:00 pm of the same day. Blood extracted between 1:00 pm to 5:00 pm, results shall be made available on or before 12:00 noon of the following day.
How will I get the result?
West Metro will send the results electronically via email to walk-in patients and will send it directly to Company HR electronically for corporate accounts.
Can I buy the Rapid Test Kit in any pharmacy or drug store?
No. This Test kit is not the same with that of Pregnancy Test that can be purchase in pharmacy. Only licensed Facility can administer the Rapid Test like Hospitals.
FDA Approved
Out test kits are 100% tested and approved by the FDA